Measurement of drug concentrations in biological matrices is an important aspect of biologics development. Pharmacokinetics/Pharmacodynamics (PK/PD) data from pre-clinical and clinical studies are required to support applications for both new biologics and biosimilar products. PK/PD relationship results are used to make critical decisions supporting the safety and efficacy of a product in development. It is therefore paramount that the applied bioanalytical methods used are well characterized, fully validated and documented to a satisfactory standard in order to yield reliable results.
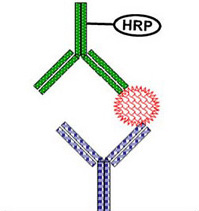 Bioavailability measurement of biotherapeutics is more often than not complicated by their size, heterogeneous nature, presence of endogenous counterparts,and the presence of anti-drug antibodies. The classical analytical methodologies used for quantification of small molecules usually fail to produce reliable data for biotherapeutics. Therefore, ligand-binding assays (LBA) are commonly used for their levels quantification.
Bioavailability measurement of biotherapeutics is more often than not complicated by their size, heterogeneous nature, presence of endogenous counterparts,and the presence of anti-drug antibodies. The classical analytical methodologies used for quantification of small molecules usually fail to produce reliable data for biotherapeutics. Therefore, ligand-binding assays (LBA) are commonly used for their levels quantification.
To ensure that an assay can be used in the quantification of a biologic drug product and withstand regulatory scrutiny, specific assay components need to be evaluated, subsequently validated, and continually monitored. Just to name a few, specificity of reagents (capture antibody), matrix effect, sample stability, must be assessed, and incurred sample reanalysis must be planned for the study.
A PK assay life cycle can be categorized into 3 general phases: method development, pre-study validation, and in-study validation. Therefore, validation is a continuing process as long as the assay is in use.